Our mission and passion is to improve the lives of cancer patients. We develop novel, blood-based assays for patients with cancer. We are currently developing a new product named Alibrex®. Alibrex is a blood test designed to identify, in real-time, stage IV cancer patients who are experiencing disease progression. Alibrex is will be available in late 2024, early 2025.
Under the current standard of care, it takes three months before an oncologist knows if a patient's treatment is working. Alibrex is a real-time therapy monitoring system that allows oncologists to assess whether a patient's treatment is working in just 12 days after starting treatment - regardless of tumor or therapy type. The benefits of Alibrex are:
The idea behind how Alibrex works is actually very simple. Tumors shed DNA into the blood. When tumors grow, the amount of DNA shed into the blood increases. The Alibrex system works by comparing the concentration of DNA in the blood prior to the start of treatments with the concentration of DNA in the blood after the administration of the 1st cycle of treatment. If the concentration of DNA in the blood increases, it is indicative of patient's tumor growing because the cancer treatment is ineffective, or the tumor is not responding to treatment.
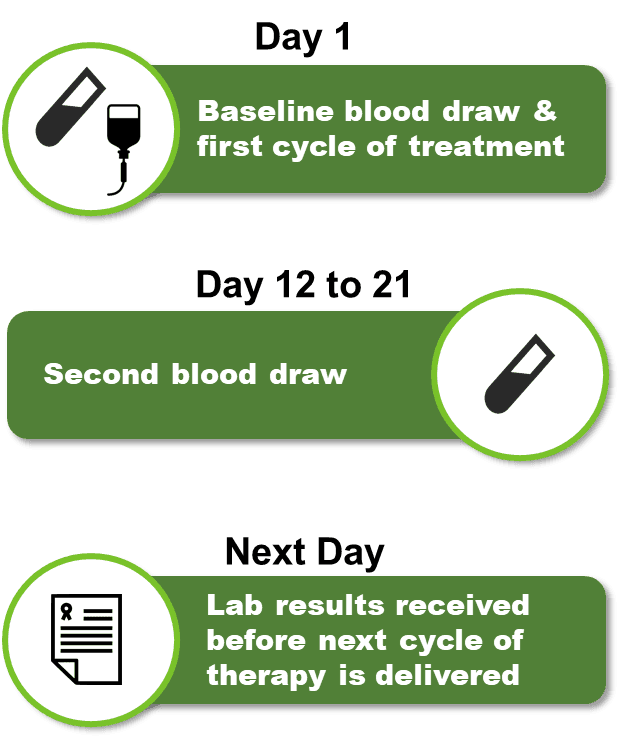
Our abilitity to detect extremely small changes in the quantity of DNA in the blood enables us to assess the treatment effectiveness after only 12 days from the first dose of therapy. Because the turn-around time of running our test is usually less than a day, we can usually get an answer back to the physician before the patient has been given a second cycle of therapy, in other words, in real time!
This proof-of-concept study was conducted to determine if our technology was able to distinguish small fragments of DNA between cancer and non-cancer patients and between cancer patients who have been treated versus those who have not been treated. With an area under the curve (AUC) of 0.989, the results of this study were positive.
This concordance study compared Alibrex results at 14 to 28 days against RECIST results at 9 to 12 weeks. A positive test outcome indicates the study participant's disease progressed (PD), a negative test outcome indicates the participant's disease did not progress (non-PD). The results of this study were positive. Alibrex was able to predict 63% of participants with PD or non-response to therapy. Most critically, there were no false-positive results.
This concordance study compared Alibrex results at 12 to 21 days against imaging results at 9 to 12 weeks. A positive test outcome indicates the study participant's disease progressed (PD) or non-response to therapy, a negative test outcome indicates the participant's disease has not progressed (non-PD), or responsive to therapy. The results of this study were positive.
The CADEX-0001 results showed an area under the curve (AUC) of 0.93 when Alibrex (at 12 to 21 days) is compared against imaging (at 9 to 12 weeks).
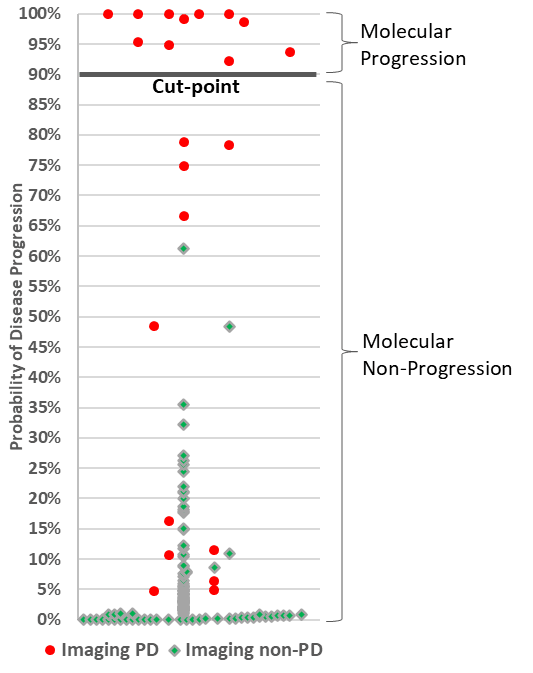
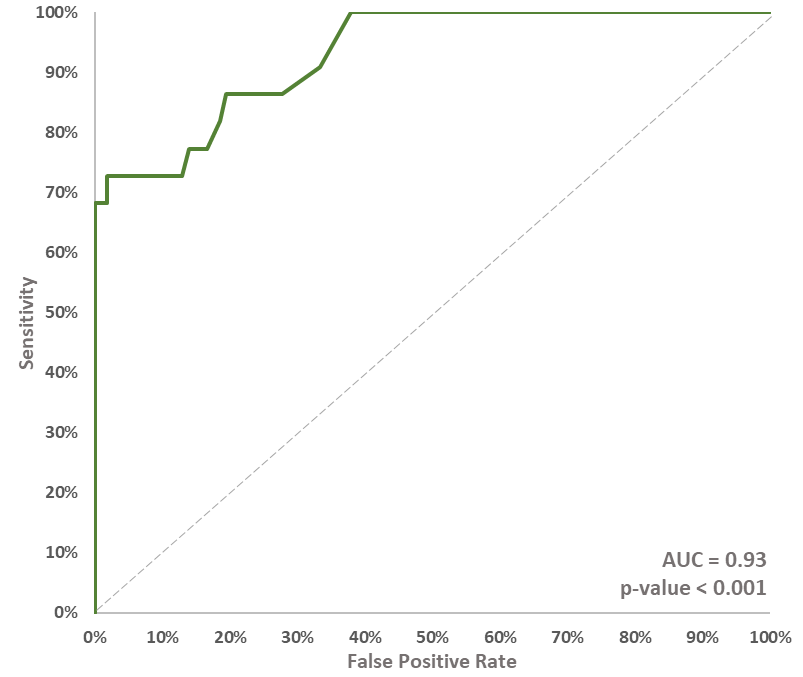
Brief description of CADEX-00014: